Development Pathway
Let’s Work Together
At Dr. Hotha’s Life Sciences LLC, we’re dedicated to simplifying the complexities of drug development through our Partner, Plan, Prosper approach. Contact us today to see how we can help you transform complexity into clarity and achieve faster, more efficient market entry.
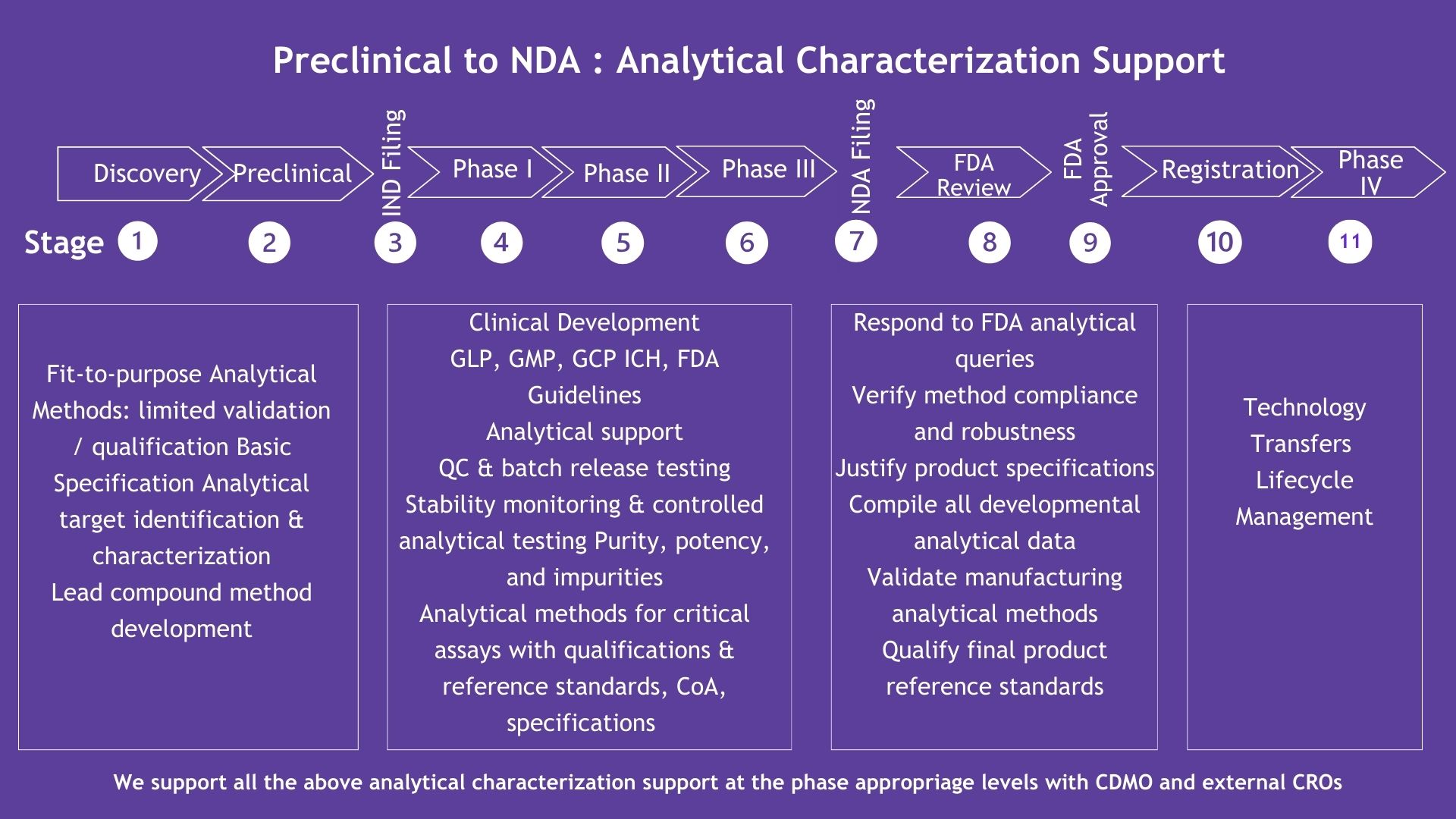
Preclinical to NDA – End-to-End Consulting Services
Comprehensive Consulting Across the Drug Development Lifecycle
At Dr. Hotha’s Life Sciences LLC, we guide our clients through every phase of drug development, from preclinical stages to NDA submission and commercialization. Our comprehensive consulting services ensure that you have the right expertise at every stage, streamlining processes, accelerating timelines, and ensuring regulatory compliance. We focus on providing strategic insights for CDMO selection, CMC planning, fast-to-clinic strategies, and regulatory submission support to help your product reach the market successfully.
Preclinical to NDA – Analytical Characterization Support
Analytical Characterization: Ensuring Quality and Compliance
Our analytical consulting services provide critical support to your drug development programs, from preclinical stages to regulatory submission. We ensure that analytical methods are fit-for-purpose, meet all necessary quality standards, and are aligned with global regulatory guidelines. From method development to QC testing and stability monitoring, we ensure that your product’s analytical profile is robust, reliable, and compliant with FDA and other regulatory bodies.
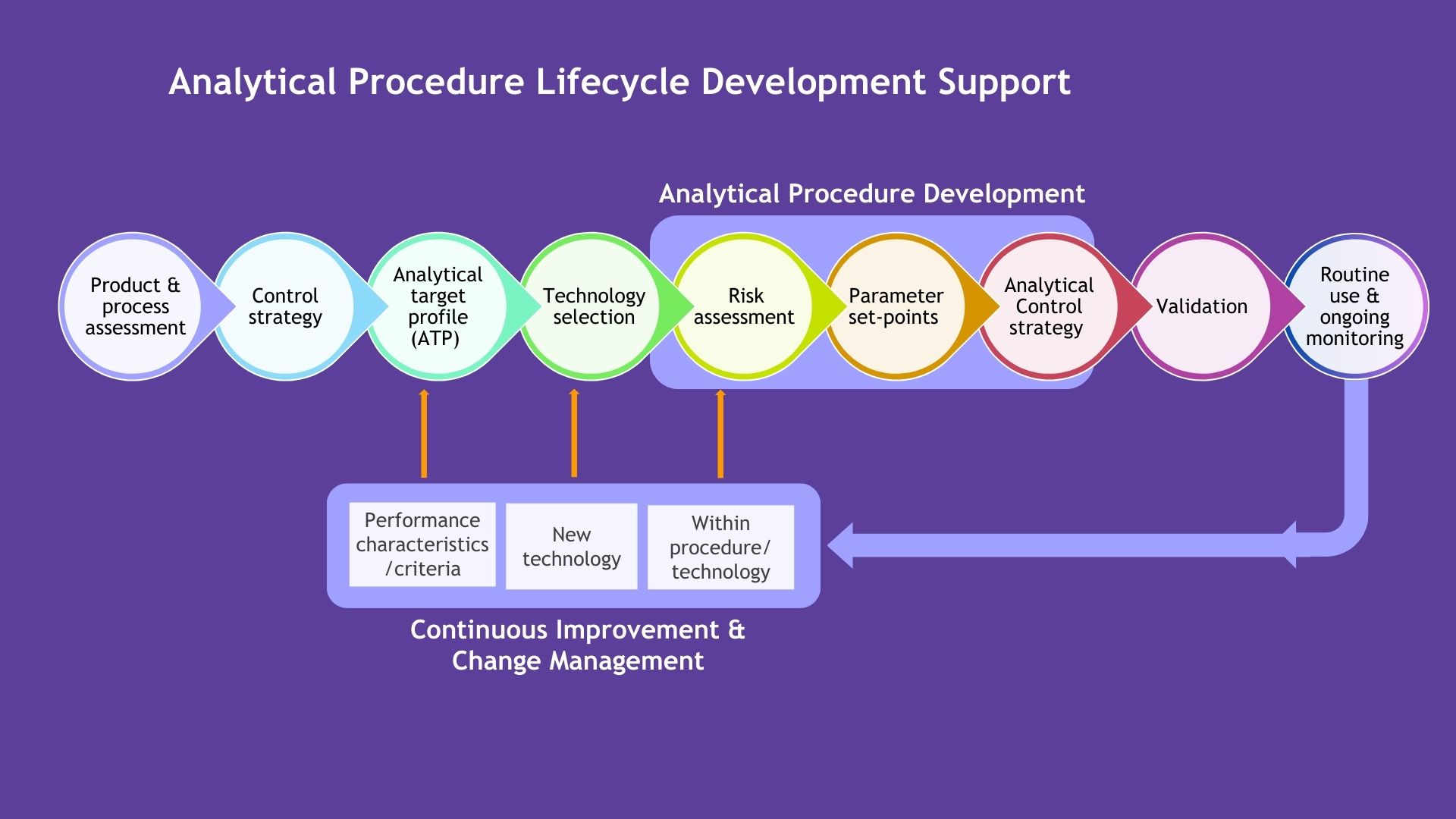
Analytical Procedure Lifecycle Development Support
Lifecycle Management for Analytical Procedures
Managing analytical procedures throughout their lifecycle is essential to maintaining regulatory compliance and product efficacy. We provide comprehensive support for the development, validation, and continuous improvement of analytical methods. Our expertise in control strategies, new technology integration, and risk assessments ensures that your analytical procedures evolve with your product, from early-phase development to routine commercial use. By focusing on continuous improvement and change management, we help you optimize performance and maintain compliance at every stage.
Partner with Us for End-to-End Development Solutions
Contact us today to see how we can help you navigate the complexities of drug development and accelerate your path to market.
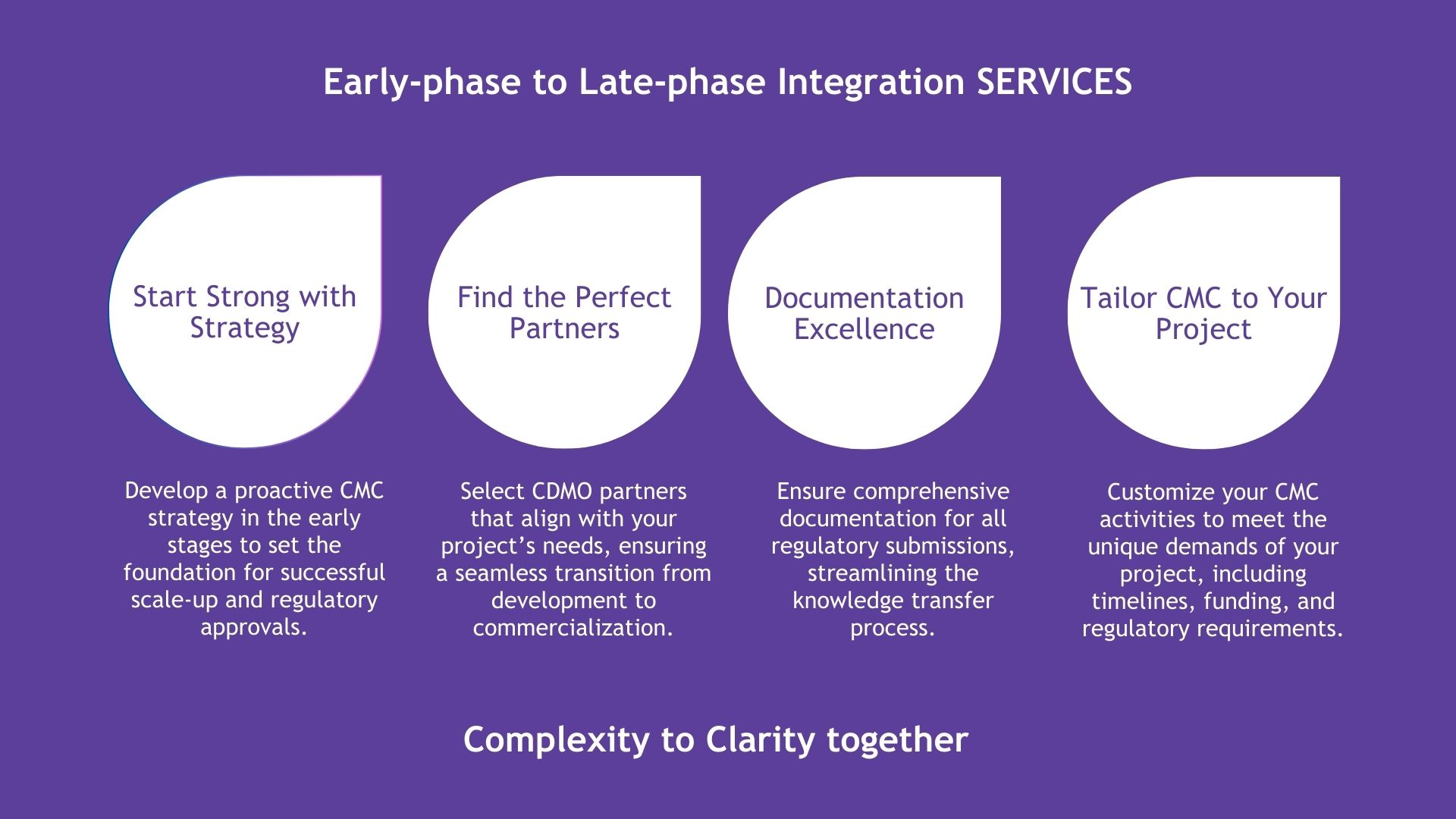
Introduction to Early-Phase to Late-Phase Integration Services
In the journey of drug development, transitioning smoothly from early-phase research to late-phase commercialization is critical for success. At Dr. Hotha’s Life Sciences LLC, we understand the complexities involved in scaling up and navigating the regulatory landscape. Our Early-Phase to Late-Phase Integration Services are designed to ensure that your drug development process is not only compliant but also optimized for efficiency and success.
Why Integration Matters: Successful drug development requires more than just scientific expertise; it demands strategic planning, precise execution, and seamless integration of every phase. By establishing robust strategies early, aligning with the right partners, meticulously documenting processes, and customizing activities to meet regulatory and commercial demands, we help you avoid common pitfalls and accelerate your path to market.
Explore Our Services: As we delve into the specifics of our services on the following slide, you will see how our integrated approach can bring clarity to complexity, transforming challenges into actionable strategies that lead to successful outcomes.