Reinventing the Biotech Core Team
As numerous CDMOs race to expand and showcase technical strengths, sustaining differentiation has become increasingly difficult. At the same time, a biotech funding crisis is reshaping collaboration priorities, demanding leaner, more integrated teams capable of trust-driven execution and shared risk.
Panel Discussion PROTACs
Targeted Protein Degradation (TPD) represents a paradigm shift in drug discovery by leveraging cellular proteostasis mechanisms to remove disease-relevant proteins instead of merely inhibiting them. Unlike traditional small-molecule inhibitors, TPD strategies—such as PROTACs and molecular glues—enable the catalytic degradation of previously undruggable targets. These modalities are rapidly advancing through preclinical and clinical pipelines, with over 20 degraders currently in trials and applications that span oncology, immunology, neurodegeneration, and rare diseases, along with increasing uses.
ICH & FDA APPROACH TO CRITICAL QUALITY ATTRIBUTES
The development of pharmaceutical products is a complex, highly regulated process that requires meticulous attention to detail to ensure the final product's safety, efficacy, and quality. Critical Quality Attributes (CQAs) are fundamental to this process, serving as benchmarks defining a drug product's quality. This white paper explores the concept of CQAs, their importance in drug development, and the methodologies used to identify, monitor, and control them throughout the product lifecycle. By understanding and effectively managing CQAs, pharmaceutical companies can enhance product quality, comply with regulatory requirements, and ultimately deliver safe and effective therapies to patients.
AI and ESG in Pharma and Biotech: Navigating the Intersection for a Sustainable Future
The pharmaceutical and biotech sectors are at a pivotal moment where integrating Artificial Intelligence (AI) and Environmental, Social, and Governance (ESG) initiatives is no longer optional but necessary. As global supply chains grow increasingly complex, stakeholders—from consumers to investors—demand transparency, ethical practices, and sustainability. In parallel, AI is being celebrated as a transformative tool to achieve these goals. But how do these forces converge, and what challenges and opportunities do they present for the pharmaceutical and biotech industries?
Scaling Innovation: How Technology Transfers Bridge Innovation and Commercialization in the Biotech and Pharma Industry
Technology transfers are pivotal for bridging innovation and commercialization in the biotech and pharma sectors. By ensuring smooth transitions from lab-scale research to large-scale production, they facilitate compliance and scalability for advanced modalities, including personalized medicine and biologics. With AI-powered predictive models, digital twins, and modular manufacturing, CDMOs enhance process robustness and expedite regulatory approval, driving the industry toward efficient, sustainable, and patient-focused drug development.
Is Your CDMO Using These Aidriven Solutions in CMC For Oliogs and Peptides?
Artificial intelligence (AI) is revolutionizing biomanufacturing by addressing critical challenges, especially in scaling production. AI platforms anticipate and resolve bottlenecks by analyzing vast data sets to detect variability in real time. For example, AI-powered monitoring systems flag deviations in synthesis or purification processes, allowing immediate corrective actions.
Rethinking Nitrosamines – Moving Beyond Compliance
The detection of nitrosamines became a priority after contamination in drugs like sartans, ranitidine, and metformin led to recalls starting in 2018.Nitrosamines are classified as probable human carcinogens (Group 2A) by the IARC. High-profile recalls have triggered global scrutiny, leading to enhanced guidelines by the FDA, EMA, and other agencies.
Where Biotech Wins: “The Role of CMC Excellence”
The development of pharmaceuticals, particularly in biotechnology, hinges on the successful execution of Chemistry, Manufacturing, and Controls (CMC) strategies. These strategies form the backbone of product quality, regulatory compliance, and operational efficiency, yet they are fraught with challenges that can derail timelines, inflate budgets, and impede market access.
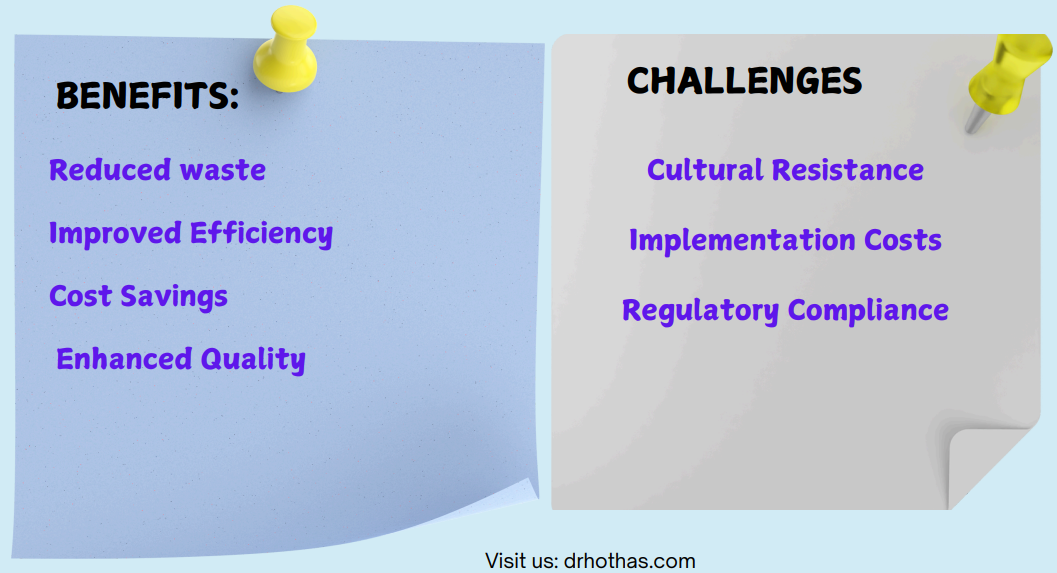
Operational Excellence Tools in Pharma & Biotech for Driving Efficiency
Operational excellence is at the heart of transforming pharmaceutical and biotech industries by ensuring streamlined processes, minimizing waste, and improving quality. This document introduces key methodologies and tools, such as Lean, Six Sigma, BPM, Automation, and Supply Chain Optimization, to drive efficient workflows, meet regulatory compliance, and deliver cost-effective solutions.
Research & PublicationsDr. Kishore Hotha2024-09-14T19:08:13+00:00